Introduction: Follicular lymphoma (FL) comprises 15% of all lymphoma cases and 70% of indolent lymphomas. Cytotoxic, targeted, and novel treatments lead to prolonged remission periods, with a median overall survival (OS) of greater than 10 years. For this reason, surrogate endpoints for OS are frequently used in clinical trials of FL to allow for quicker results and shorter time to drug approvals, as well as to offset the likely cost and difficulty in funding long term trials with OS as a primary endpoint. A frequently used surrogate endpoint is progression free survival (PFS); however, PFS has not been previously validated as a surrogate endpoint for OS in the treatment of FL. Previous systematic reviews of multiple trials did not have mature enough data to draw definitive conclusions (Zhu et al, AAPS J 2017).
Methods: To evaluate the correlation between PFS and OS in FL trials, we conducted a comprehensive systematic review. We included all completed phase 2 or 3 clinical trials with posted results on ClinicalTrials.gov from the inception of the database until July 2023. Additionally, we manually added trials that were completed before the database inception in February 2000 culled from HemOnc.org. For inclusion, randomized controlled trials reporting a hazard ratio for both PFS and OS were considered. We performed statistical analyses, utilizing unadjusted linear regression weighted by sample size to evaluate the association between hazard ratios for PFS and OS.
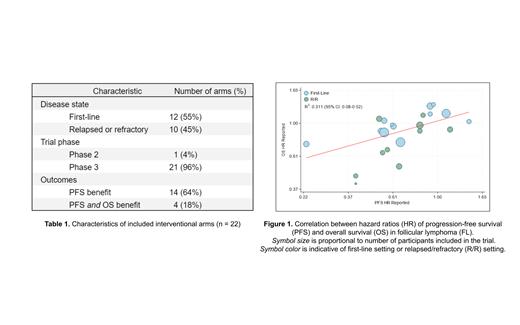
Results: A total of 169 randomized controlled trials for FL were initially identified. Upon screening, 74 randomized trials reported rates for both PFS and OS. 20 trials with 22 interventional arms that included hazard ratios were eligible for inclusion in final analysis. 21 (96%) phase 3 trials and 1 (4%) randomized phase 2 trial were included, encompassing 10,729 participants. Among the included interventional arms, 12 (55%) were in the first-line setting and 10 (45%) were in the refractory or relapsed setting. 14 (64%) arms demonstrated a PFS benefit, and 4 (18%) demonstrated both a PFS and OS benefit. Disease characteristics and treatment outcomes are included in Table 1. The correlation coefficient between PFS and OS was 0.558 (95% CI 0.545-0.571), indicating a weak association. The coefficient of determination was 0.311 (95% CI: 0.08-0.52) suggesting 31.1% of OS variance could be explained by changes in PFS (Figure 1).
Discussion: PFS showed a weak correlation with OS in FL trials, challenging its role as a surrogate endpoint. Factors such as patient heterogeneity, treatment regimens, subsequent therapies, and treatment-related complications may contribute to the discrepancy. While PFS aids regulatory approval and drug development, our study suggests that PFS may not be an appropriate surrogate endpoint for OS in the treatment of FL, and alternative surrogate endpoints should be pursued. Possible alternative endpoints though will need to be validated using similar systematic methods given often contradictory findings between individual studies. For example, progression within 24 months of initial treatment (POD 24) was a valid surrogate endpoint for OS in the PRIMA study, however this was not the case in the GALLIUM study (Bachi et al., Blood Adv 2021). Other surrogate endpoints such as complete response rate at 30 months (CR30) have demonstrated strong correlation with PFS however their association with OS has yet to be established (Shi el, JCO 2016).
Disclosures
Ollila:ADC Therapeutics: Honoraria; Ono Pharmaceuticals: Honoraria, Research Funding. Olszewski:Genmab, Blue Cross/Blue Shield of Rhode Island, Schrodinger, ADC Therapeutics, BeiGene: Consultancy; Leukemia & Lymphoma Society, Genetech, Inc. / F. Hoffmann-La Roche Ltd, Adaptive Biotechnologies, Precision Biosciences, Genmab: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal